In the realm of pharmaceuticals, ensuring patient safety, optimizing regulatory processes, and fostering global collaboration are paramount. Enter IDMP, an acronym that stands for Identification of Medicinal Products. While...
When submitting general medical devices for approval in the ASEAN region, it is essential to follow the guidelines and standards set by the ASEAN Common Submission Dossier Template (CSDT)...
The revised EDQM guideline “content of the dossier” and other key documents will be useful tools With effect from May 1, 2024, the updated EDQM guideline “Content of the...
In the world of business, certain identifiers hold significant importance, and one such identifier is the DUNS number. Short for Data Universal Numbering System, a DUNS number is a...
In the ever-evolving landscape of pharmaceutical regulations and submissions, keeping pace with the latest standards is crucial. One such standard that has gained prominence in recent years is the...
In today’s regulatory environment, maintaining accurate and accessible documentation is crucial for compliance. Document Management Systems (DMS) play a vital role in ensuring that organizations can meet regulatory requirements...
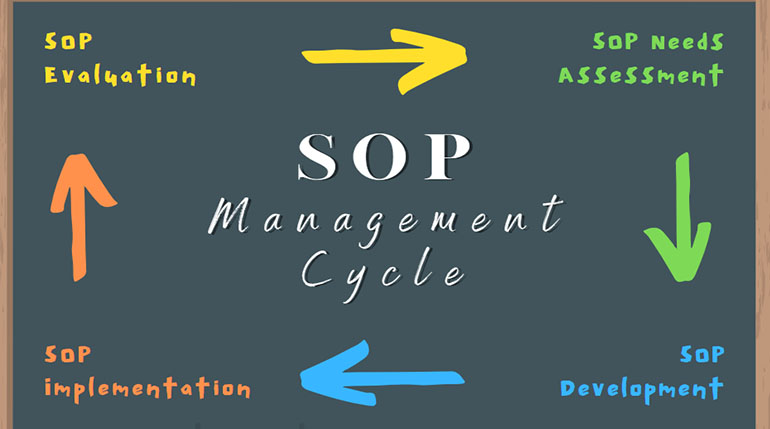
Standard Operating Procedures (SOPs) are the backbone of any effective Quality Assurance (QA) program. They provide clear instructions for performing tasks consistently and correctly. In this blog post, we’ll...