Post-Approval Variation (PAV) in the USA refers to changes made to an already approved drug application (e.g., NDA, ANDA, or BLA) after it has received marketing authorization from the...
When implementing the eCTD (Electronic Common Technical Document) in Tunisia, there are key aspects to consider for successful submission to the local health authority, the Direction de la Pharmacie...
The electronic Common Technical Document (eCTD) has become the global standard for submitting regulatory information to health authorities, streamlining the process for pharmaceutical and biotechnology companies. While the basic...
In the complex world of drug development, navigating regulatory pathways efficiently can determine how quickly and successfully a product reaches the market. One such pathway in the U.S. is...
TFDA eCTD Specification : The Taiwan Food and Drug Administration (TFDA) has recently released its guidelines for the electronic Common Technical Document (eCTD) format, aiming to standardize and streamline...
This document is intended to guide the submission of a product registration dossier to HSA via the Medical Device Information and Communication System (MEDICS). This guide specifies the appropriate...
The PRIME scheme aims to support the development of novel medicines that target an unmet clinical need in conditions that are seriously debilitating or life-threatening. The scheme offers enhanced support to...
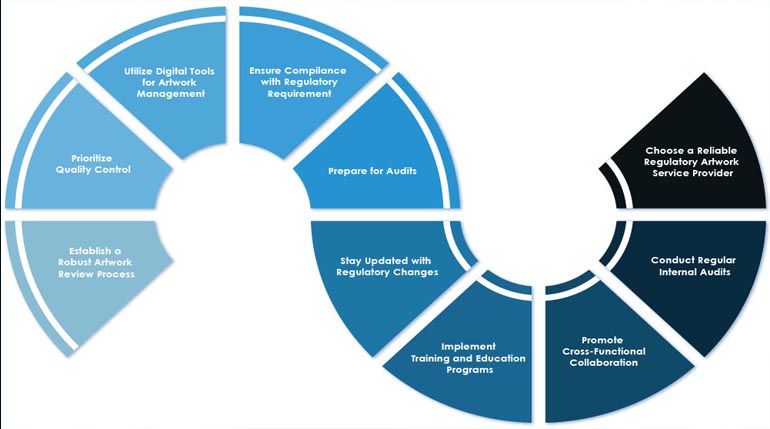
The technical and regulatory aspects of drug development and production, encompassing the chemical composition of the drug substance, its manufacturing process, and the controls implemented to ensure its quality...
In the fast-paced world of pharmaceuticals, maintaining quality and compliance is of utmost importance. One crucial aspect of the drug development process is the Drug Master File (DMF) writing guideline. The DMF serves...
The revision 1 of the MDCG 2019-07 Guidance on Article 15 of the Medical Device Regulation (MDR) and In Vitro Diagnostic Device Regulation (IVDR) on #PRRC has been published in December...