In the ever-evolving landscape of pharmaceutical regulations and submissions, keeping pace with the latest standards is crucial. One such standard that has gained prominence in recent years is the...
In today’s regulatory environment, maintaining accurate and accessible documentation is crucial for compliance. Document Management Systems (DMS) play a vital role in ensuring that organizations can meet regulatory requirements...
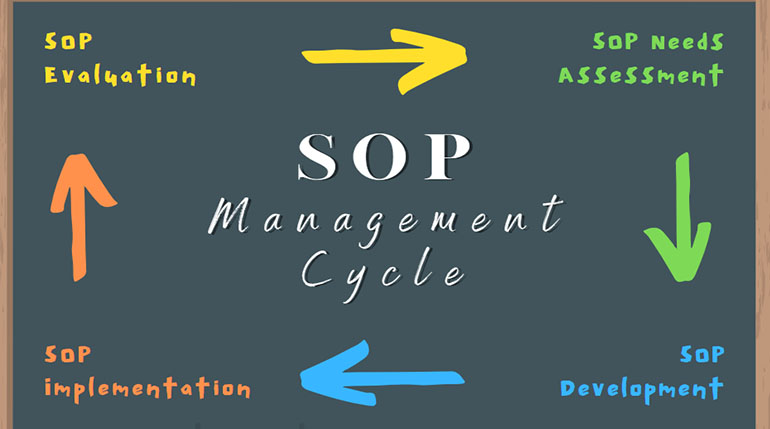
Standard Operating Procedures (SOPs) are the backbone of any effective Quality Assurance (QA) program. They provide clear instructions for performing tasks consistently and correctly. In this blog post, we’ll...